Artificial Cochlea has become a critical medical device for individuals suffering from single-sided deafness or hearing impairment
Acoustic neural stimulation, also
known as the artificial cochlea, is an electronics device that transmits a
predetermined signal of a sound to a person severely hearing impaired by
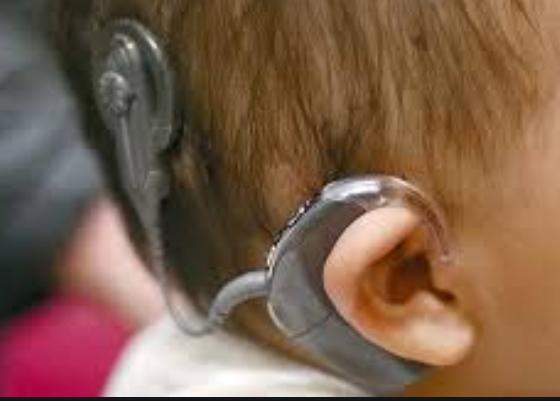
hearing loss. The implant generally consists of a thin external part that sits
behind your ear and another surgically located part underneath the skin. The
signals transmitted by the artificial cochlea are then converted by the inner
ear into a sound. This allows the individual to hear normally again. As per the
JapanTark study, around 12.8% of the adult Japanese population suffer from
hearing loss.
There are many types of artificial
cochlea based on the degree of hearing impairment. One such type is the
piezoelectric cochlea. The piezoelectric device generates a specific sound wave
by the vibrations that are formed inside it when it is hit with sound waves.
The outer skin of the device has numerous microscopic plates that generate
vibrations when hit by sound waves. These vibrations excite the cells present
in the cochlea and they produce an electrical charge that changes the current
flowing in the cochlea. Cochleae allow auditory signals to flow to the brain,
which processes them. With the cochlea positioned correctly, they can direct
sounds and produce other vibrations that tune the auditory system.
This technology makes use of the
latest technology that is being used by hearing-aid companies. This includes
the transducers that can be placed near the auditory nerve to directly
stimulate the nerve. Then again, there are many people who believe that the
transducers can also directly stimulate the auditory nerve, resulting to a
placebo effect. Nevertheless, many researchers see the potential of the
technology and predict that in the near future, we will be able to use the
transducers to stimulate nerves and even treat some forms of hearing loss
directly. Recently, in March 2021, Cochlear Limited received the U.S. Food and
Drug Administration (FDA) approval for its new Cochlear Baha 6 Max Sound
Processor designed for people with single-sided deafness.



Comments
Post a Comment