What is electroplating? How does the electroplating process work?
Electroplating is the
way toward applying a metal covering on another piece of metal (or another
conductive surface) through an electro-testimony measure. In electroplating,
the saved metal turns out to be essential for the current item with the
plating/covering.
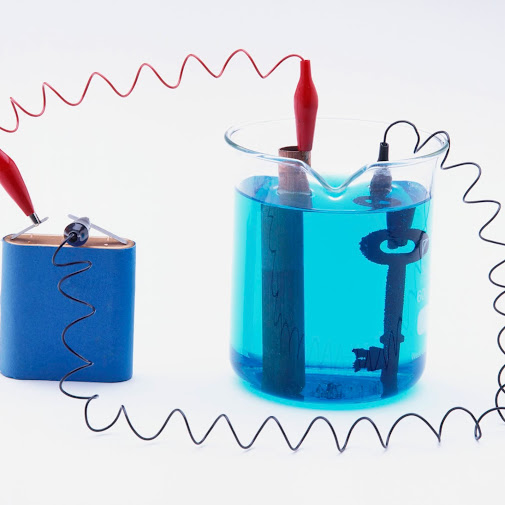
In Electroplating, both an anode and a cathode (the metal part to be
covered) are submerged in an electrolytic shower that is made out of an answer
of salts, including the metal to be plated. An immediate flow (DC) of power is
gone through the arrangement, affecting the exchange of metal particles onto
the cathodic surface, plating the metal onto the thing.
In electroforming, the
mandrel (designed substrate) will be taken out from the item. After the mandrel
is eliminated, the article that remains is made through electro-testimony.
Subsequent to electroforming, it is feasible to perform electroplating to add a
covering to improve erosion obstruction or to get a more appealing (corrective)
item. So as opposed to the two strategies being utilized autonomously, they can
really be utilized in an agreeable way.
A straightforward
illustration of the electroplating cycle is the electroplating of copper
wherein the metal to be plated (copper) is utilized as the anode, and the
electrolyte arrangement contains the particle of the metal to be plated (Cu2+
in this model). Copper goes into arrangement at the anode as it is plated at
the cathode.
Read More : https://bit.ly/3tjwIOG



Comments
Post a Comment